AMG0103 rescues spinal discs in preliminary drug trial
AMG0103 targets very specific anatomy (the nucleus pulposus of an intervertebral disc) and very specific physiology: it interferes with inflammatory degeneration of tissues in an intervertebral disc.
A small new study is a big (early) win for a new kind of medication for back pain (and probably more). For now, it’s known as “AMG0103,” a working title for this mouthful of a molecule, which is an NF-kappa B oligonucleotide decoy. Its goal is to suppress inflammation-powered tissue degeneration in spinal discs by interfering with an immune system pathway further “upstream” than other anti-inflammatory drugs: “a therapy that simultaneously targets specific actions of multiple key cytokines,” by acting “as a decoy for the NF-kB DNA binding sequence in the disc.”
In other words, AMG0103 is not just a pain blocker, but a pain solver that meaningfully reduces the excessively persistent and destructive inflammation that may be the root of many painful problems — or at least closer to the root. Not all inflammation is functional! To test this principle, the researchers gave an intradiscal injection of AMG0103 to 25 patients with presumed discogenic low back pain, right into the gooey nucleus of degenerated spinal discs.
The lucky recipients felt quite a lot better, a whopping 77% average reduction in back pain — and a median reduction of just shy of 100%! By contrast, the placebo group only managed a 40% average reduction. These results — and this is where it starts to get amazing — lasted for at least a year, from a single injection.
But wait, there’s more.
The pain reduction was probably persistent because their intervertebral discs actually regenerated — they got measurably thicker as the weeks went by. So they didn’t just feel better… they were better. This isn’t “regenerative medicine,” per se — it’s probably just solving a problem that was blocking normal healing — but it might as well be. Cool. Very cool.
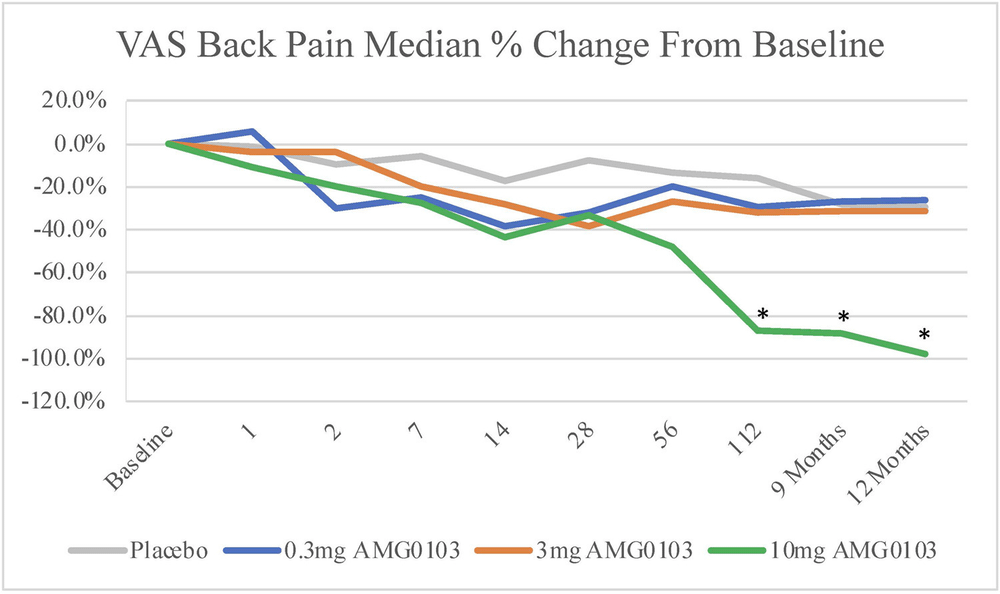
Pain was measured over time on a simple 1-10 scale for three different doses of AMG0103 (blue, orange, green for small, medium, large) and a placebo. The small and medium doses didn’t do much compared to placebo, but that green line ends up in a very good place.
What could possibly go wrong? About safety
It’s reasonable to be concerned about a drug that works by suppressing some immune function. “With great power” and all that.
AMG0103 fights inflammation, but it is not “immunosuppressive” in the usual sense: it’s too precise, both physiologically and anatomically. It modulates inflammation in a specific spot in a specific way, mainly interfering with the tissue catabolism (“stop dismantling that!”), such that tissue gets shored up instead — which is the opposite of the ugly tradeoff we see with steroid injections, which ease pain (maybe) at the expense of being downright corrosive to tissues.
The study was looking for signs of immune-related dysfunction, but found none. In fact, the only adverse event reported was one case of brief, mild nausea:
Throughout the 1-year follow-up, patients did not experience clinically relevant organ dysfunction or neurologic complications at any of the 3 therapeutic doses delivered
Some local adverse effects cannot be ruled out yet, but so far the safety profile looks just as promising as the efficacy.
Preliminary, yes, but it’s such a good kind of preliminary
The glaring caveat here is that this was a tiny study of just a handful of ideal subjects in a phase 1B study, an early clinical trial exploring both safety and efficacy on a small scale. “One-bee” 🐝 trials are for subjects with a relevant condition that actually needs treating (having previously established safety and tolerability in completely healthy volunteers, which is 1A). They are first trial in the process that can be taken seriously as “promising” — but they also cannot be more than that. “Clinically proven” is miles away.
But they can be more or less promising, depending on the effect size … and this effect size was bonkers. It was not just clinically “significant,” it was clinically dramatic.
And a successful 1B for AMG0103 also constitutes a valuable proof of concept. The mechanism of action is based on a solid and important hypothesis about the nature of the beast — exactly the kind of thing we generally want to see in pain science, but rarely have. The result was highly plausible and specific, which really protects the dramatic results from being dismissed as too good to be true.
It’s the principle of the thing
This trial’s strong results are good news in principle, even if there is some clinical deal-breaker for AMG0103 yet to be identified. It provides good support for the hypothesis that back pain is a lot more about bio-chemistry than the traditional bio-mechanical scapegoats, or the more progressive and fashionable bio-psychosocial scapegoats. Most care for chronic somatic pain is based on mechanical and/or psychosocial ideas about how pain works:
- The biomechanical paradigm, or “structuralism,” puts too much emphasis on the role of things like dysfunctional posture, movement, and anatomy (e.g. “short legs” that aren’t actually short enough to cause a problem).
- The biopsychosocial paradigm is based on hypothesis that much persistent pain is often a problem with pain itself (“nociplastic” pain), which gets entrenched and “amplified” by psychology, perception, and cautious movement.
Neither paradigm is useless, of course, but both can (and have) been strongly criticized for barking up the wrong trees, and for neglecting the huge role of pathophysiology, for only paying lip service to the “bio” … because there isn’t much “common sense” to be found that direction, and also probably because neither of these paradigms can do anything about it. Pathophysiological problems are mostly going to be solved by scientists in labs, not body mechanics in clinics.
The success of AMG0103 in this trial supports the idea that the right tree to bark up is the biochemistry tree: a lot of chronic pain is probably mostly a function of chronic inflammation, and the challenge is to figure out how to stop it without shutting down the whole immune system.
I’m not excited, but I am certainly intrigued
I haven’t been truly excited about anything in this field for 20 years, and I am not starting today. AMG0103 is cool, but getting excited about it would be like getting excited about a half-baked loaf of bread — it’s just not ready! Of course more study is needed — as for practically anything that isn’t rank quackery.
But I am certainly happy to report a strikingly positive trial result, with a truly noteworthy effect size, and meaningful implications for how a very common kind of pain actually works. And this is that.